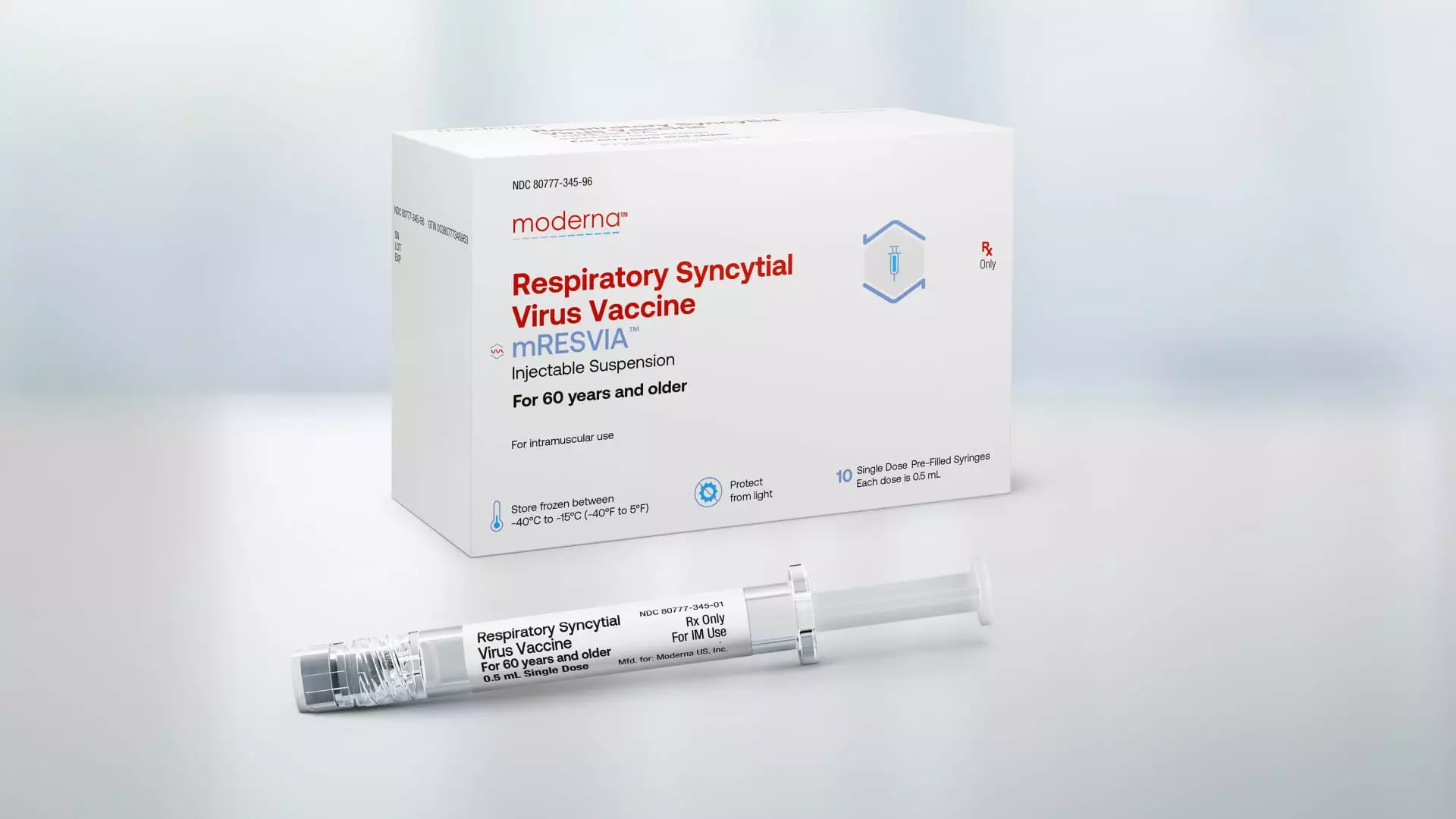
In a recent announcement, the Food and Drug Administration granted approval for Moderna’s vaccine for respiratory syncytial virus (RSV) for adults aged 60 and older. This marks a significant milestone for Moderna, as it diversifies its product portfolio beyond its Covid jab, which has seen a decrease in demand. The decision to approve Moderna’s RSV vaccine was based on a late-stage trial involving older adults, who are at a higher risk of severe RSV cases.
RSV is a serious virus that claims the lives of thousands of seniors each year and leads to tens of thousands of hospitalizations. Moderna’s vaccine, marketed under the name mRESVIA, is the first messenger RNA vaccine to be approved for a disease other than Covid. This vaccine is also unique in that it is available in a pre-filled syringe, making it more convenient for healthcare providers to administer to patients. The approval of Moderna’s RSV vaccine highlights the company’s commitment to addressing global public health threats.
With the approval of Moderna’s RSV vaccine, the company’s revenue prospects are expected to improve. Moderna’s full-year 2024 sales guidance includes revenue from its RSV vaccine, which is estimated to contribute significantly to the company’s overall revenue. The positive recommendation from the CDC for the use of Moderna’s vaccine will allow it to compete with other RSV vaccines from GSK and Pfizer, both of which have been on the market for some time. Moderna’s innovative mRNA platform demonstrates its potential to develop vaccines for a range of diseases, including cancer and infectious diseases like norovirus.
Looking ahead, Moderna has a robust product pipeline with over 40 products in development, including a combination shot targeting Covid and the flu. The company’s goal is to return to sales growth by 2025 and break even by 2026, driven by the launch of new products. Investors are optimistic about the long-term potential of Moderna’s mRNA product pipeline, as evidenced by the significant increase in the company’s stock price in recent years. Moderna’s mRNA platform has the potential to revolutionize the way we approach infectious diseases and other health challenges.
The FDA’s approval of Moderna’s RSV vaccine is a significant achievement for the company and a positive development for public health. Moderna’s innovative mRNA platform has the potential to revolutionize the vaccine landscape and address a wide range of health challenges. With a strong product pipeline and a commitment to research and development, Moderna is poised to make a lasting impact on global public health.